Generic drug
K.Thirugnana Sambanthan |Assistant Professor
Sankara Institute of Management Science(SIMS)

In the present era, many people are infected with new diseases. The cost of the drugs are becoming high for several dreadful diseases. The common man is finding it difficult to buy these life saving drugs. The cost of a cancer drug is around Rs.1.5 Lakhs, where as the same equivalent drug from another company is just Rs.8,500. Most of the patients are buying the prescribed drugs in the recommended shop by the doctor. Pharmaceutical firms spend huge amounts in creating these brands. However, since prescription-based medicines cannot be promoted through advertisements, companies often push these brands through doctors and chemists. Consumers, who are often unable to make an informed choice for purchasing medicines, have to rely on the doctor’s prescription or on chemists.
A generic drug is a pharmaceutical drug that is equivalent to a brand-name product in dosage, strength, route of administration, quality, performance, and intended use. The term may also refer to any drug marketed under its chemical name without advertising, or to the chemical makeup of a drug rather than the brand name under which the drug is sold.
Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries where they are dispensed. They are labeled with the name of the manufacturer and a generic nonproprietary name such as the United States Adopted Name or international nonproprietary name of the drug. A generic drug must contain the same active ingredients as the original brand-name formulation. The U.S. Food and Drug Administration (FDA) requires that generics be identical to, or within an acceptable bioequivalent range of, their brand-name counterparts with respect to pharmacokinetic and pharmacodynamic properties.
Biopharmaceuticals such as monoclonal antibodies differ biologically from small molecule drugs. Generic versions of these drugs, known as biosimilars, are typically regulated under an extended set of rules.
The Indian government began encouraging more drug manufacturing by Indian companies in the early 1960s, and with the Patents Act in 1970. The Patents Act removed composition patents for foods and drugs, and though it kept process patents, these were shortened to a period of five to seven years. The resulting lack of patent protection created a niche in both the Indian and global markets that Indian companies filled by reverse-engineering new processes for manufacturing low-cost drugs. The code of ethics issued by the Medical Council of India in 2002 calls for physicians to prescribe drugs by their generic names only.
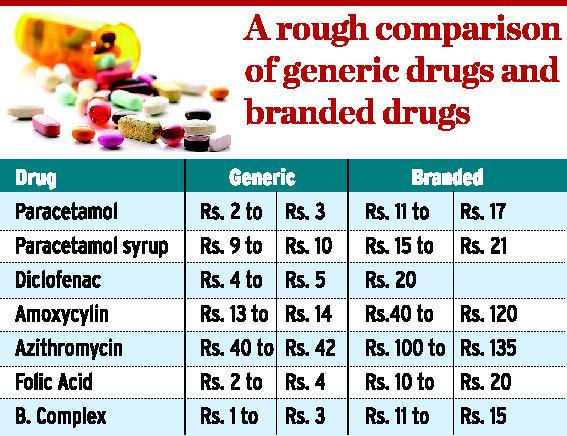
The Indian government’s latest move providing an umbrella brand for generic drugs is aimed at enabling consumers to make that choice. The consumer can walk to an chemist and ask for a ‘Jan Aushadhi’ brand for the prescribed medicine, with the government set to launch its own brand to sell low cost generic medicines. This scheme is the cure for rising medicine prices. A rough comparison of commonly used branded drugs and generic drugs is shown in the figure below.
Any one who want to buy cheap medicine. Actually poor people are most benefited through Generic Medical Store. Prior that people don’t have choice to buy economical medicine. Now poor people and retired personnel can buy life saving drug through Generic Medical Store.
Helpline number for more information about Jan Aushadhi Generic Drug Store contact on this number 1800 180 80 80.
